Expanding Sample Libraries Tops the Wish List for Compound and Sample Managers
Add bookmark
Successful drug discovery depends on having efficient compound management systems in place, which means access to a growing library of high quality compounds and samples, and sufficient automation technology with which to handle them.
With big pharmaceutical companies increasingly looking after millions of chemical compounds and biological samples, effectively organising the logistics of storing and retrieving them and streamlining data management has never been so crucial.
Research is moving away from the widespread High Throughput Screening (HTS) approach to a more selective subset or expansion screening, which conserves valuable compounds and means screening campaigns are proving more successful as leaner, more efficient libraries are screened against the target.
Many pharmaceutical companies now outsource compound management to global firms like BioFocus, which can be of huge financial benefit, but also comes with its own logistical issues.
As compound management becomes ever more important, and ahead of the Compound & Sample Management 2012 conference being held in Amsterdam in May, Pharma IQ surveyed key members of the compound management community to gauge their current concerns and future plans.
Pressing concerns
The survey asked respondents to rate their primary concerns for their department, with the seven options covering everything from job and budget cuts, to new automation technology and data management.
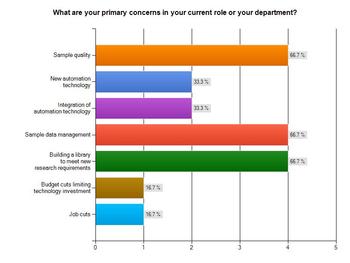
With no single priority taking precedence, the survey found that concern was equally weighted across three key issues: sample quality, sample data management and building a library to meet new research requirements, which were each selected by two thirds (66.7 per cent) of respondents.
Less of a priority, but still relevant to note, is new automation technology and its integration, both highlighted by a third of professionals as a primary concern.
But, interestingly in the current economic climate, possible job and budget cuts were not seen by many as a big worry as we look ahead to 2012, with only 16.7 per cent of respondents citing them as concerns.
Managing biological samples
To meet the growing needs of today’s drug discovery research, many compound managers are also handling biological samples as well as chemical compounds.
Half of the respondents surveyed said their sample management team or library was responsible for biological materials or samples, and half had also set up a biological sample registry. All those who managed biological samples reported that their teams solely looked after peptides, with none handling proteins, antibodies or cell lines.
Those respondents who did not have a biological registry set up were then asked to clarify why not. It appears from the results that budget to invest in a solution is not an issue, with none pointing to lack of funds as an influencer. There are instead other factors at play:
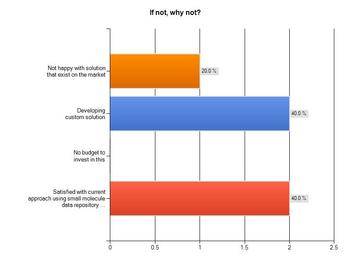
Almost half (40 per cent) said they were currently developing a custom-built solution themselves, if you add that to the 20 per cent who feel that existing solutions on the market are unsatisfactory, you could conclude that available products are falling short of most expectations.
Meanwhile, a further 40 per cent said they were happy with using small molecule data repositories and therefore not in need of a biological registry solution.
Integration issues
Bioinformatics, the application of IT to biology, has revolutionised drug development and is an integral part of managing and tracking compounds - as databases are required to store and analyse all the data from automated High Throughput and High Content Screening (HCS). Some HTS robots can test up to 100,000 compounds a day.
Respondents were asked what issues they faced in the integration of their sample information with discovery data.
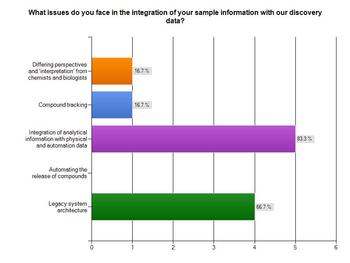
The biggest issue for most respondents (83.3 per cent) was integrating analytical information with physical and automation data, while two thirds said the legacy system architecture was an issue.
Receiving differing perspectives and interpretation of data from chemists and biologists was a less concerning issue, however, with only 16.7 per cent citing it.
Compound tracking only affected 16.7 per cent of respondents too, whereas automating the release of compounds was not seen as an issue at all.
[inlinead]
Sample preparation
From the survey results, it appears that labs are using various chromatography methods for sample isolation, without any clear trends emerging – in fact many respondents were unsure as to the techniques deployed by their companies.
Pharma IQ asked compound managers whether they currently use the traditional counter-current method of chromatography (CCC) for sample preparation. Half said no, whereas half of respondents didn’t know.
They were then asked if they use HPLC (High Performance Liquid Chromatography) for sample preparation. The results show that 50% of respondents use this method, while 16.7% do not employ HPLC, and one third don’t know.
|
|
|
Have Your Say Rate this feature and give us your feedback in the comments section below |



















